Background: Acute myeloid leukemia with myelodysplasia related changes (AML-MRC) a distinct clinico-pathologic subtype of AML recognized in the WHO and ICC 2022 classifications of hematolymphoid neoplasms (AML-MRC and AML-MR respectively). Compared to other subtypes, patients with AML-MRC/AML-MR have a worse prognosis and are less likely to respond to treatment. Traditionally, patients who are eligible for allogenic stem cell transplant (SCT) with AML-MRC/AML-MR are given high intensity chemotherapy (IC), such as daunorubicin and cytarabine (“7+3”), liposomal 7+3 or fludarabine+cytarabine+idarubicin (FLAG-IDA) whereas patients who are not transplant-eligible were treated with hypomethylating agents (i.e. azacitidine). Recently, azacitidine + venetoclax (aza/ven) has emerged as an important option for all AML patients over 75 years of age or with significant comorbidities who are not eligible for IC. However, the efficacy of aza/ven compared to IC for AML-MRC who are candidates for either has not been clearly established.
Methodology: We conducted a single centre retrospective review of patients diagnosed with AML-MRC/AML-MR at Sunnybrook Health Science Centre (SHSC). A list of patients with AML-MRC/AML-MR was compiled from records from the Sunnybrook Hematology Biobank, which collects specimens and clinical data from 95% of acute leukemia patients presenting to SHSC. 65 patient charts were reviewed, and 60 patients were included in the study. 3 patients were not treated at SHSC and 2 were more appropriately classified as AML with recurrent genetic abnormalities. Treatment with a “7+3” backbone or FLAG-IDA were considered ‘intensive’ regimens; treatment with azacitidine with or without venetoclax, and low dose cytarabine (LDAC), with or without venetoclax were considered ‘non-intensive.‘ ELN 2022 criteria were used to evaluate responses to therapy.
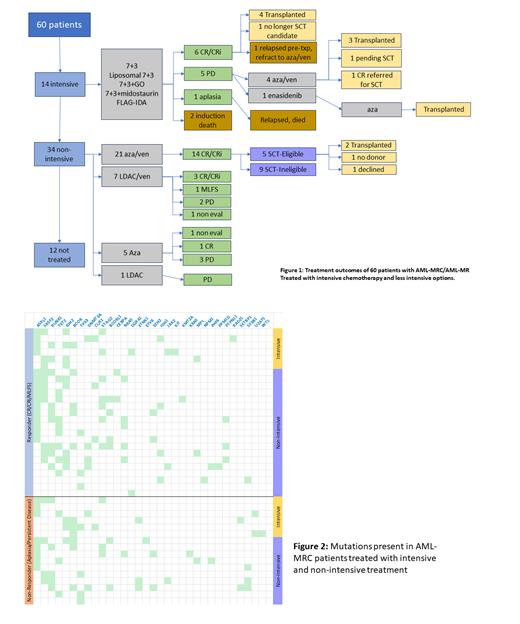
Results: 60 patients with AML-MRC/AML-MR were treated at SHSC from January 2021 to April 2023. The median age was 74 (37-99) y.o. 34 patients were male. The upper age of eligibility for allogeneic stem cell transplant was considered 72 years for this study. 28 patients were SCT eligible (31-72y.o.) and 32 patients were SCT ineligible (73-99y.o.). For SCT eligible patients, 14/28 were treated intensively and 10/28 were treated non intensively. 4 patients did not receive treatment. Of the SCT eligible group, 6/14 intensively treated patients achieved a CR/CRi compared to 5/10 non-intensively treated patients. For SCT ineligible patients, 8/32 did not receive treatment and the remaining 24 were treated non intensively. 12/24 SCT ineligible patients receiving treatment were given azacitidine+ venetoclax (aza/ven) and 9/12 achieved a CR/CRi. 7/24 patients received LDAC + venetoclax and 3/7 achieved a CR.
In total, 14/60 patients were treated intensively, 21/60 patients were treated with aza/ven, 7/60 received LDAC+venetoclax, 5/60 azacitidine alone and 1/60 LDAC alone. 12/60 patients did not receive leukemia-directed therapy, either due to concomitant illness precluding treatment or a decision to decline treatment. Notably, 14/21 patients receiving aza/ven achieved a CR/CRi compared to 6/14 intensively treated patients, though more intensively treated patients ultimately proceeded to transplant (Figure 1).
48 patients who underwent therapy had next generation sequencing data for mutations of interest in AML. The most common mutations found in this cohort were ASXL1 (32), SRSF2 (20), RUNX1 (18), TET2 (17), IDH2 (16) and TP53 (12). No differences were found between patients that responded to first line therapy compared to those that did not, or those that responded to intensive chemotherapy compared to those responding to non-intensive treatment (Figure 2).
Conclusion: Aza/Ven is an effective treatment regimen for AML-MRC/AML-MR, with 14/21 patients achieving CR/CRi after firstline treatment compared to 6/14 patients after IC. 4/4 patients achieved CR/CRi with salvage aza/ven after failing to respond to IC. Our study suggests that aza/ven should be considered as a firstline treatment option even in patients who are fit for intensive therapy as a means to achieve adequate responses to bridge to allogenic stem cell transplant without attendant toxicities associated with IC. Further studies are required to determine if specific mutations can predict response to either aza/ven or intensive treatment.
Disclosures
Buckstein:Abbvie: Honoraria; Taiho: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Tsui:Precision Dx: Consultancy; LifeLabs: Consultancy; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal